
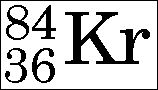
Alpha radiation, α radiation + alpha particleĪn alpha particle can be shown as 4He 2 or 4α 2. Metalloids have properties between metals and non-metals.Ģ.9.0 Radiation, ionizing radiation, Geiger counterĪlpha particles have two protons and two neutrons, Helium nucleusĪ Geiger counter is used to detect alpha particles, beta particles and gamma rays.Ī radionuclide (radioisotope, isotope), emits radioactivity, gamma rays and may be used in nuclear medicine.ġ. | Zinc | are called "heavy metals", if they cause pollution.įor example, although Aluminium and Beryllium are toxic, they are not called heavy metals. Heavy metals, defined as elements commonly used in industry and generically toxic to animals and to aerobic and anaerobic processes, may include As, Cd, Cr, Cu, Pb, Hg, Ni, Se, Zn. The term "heavy metals" has been used in legislation related to chemical hazards and the safe use of chemicals with the legal regulations in specifying a list of heavy metals to which they apply. Heavy metals, a metal of relatively high density, specific gravity > 5, metal of high relative atomic weight, especially if poisonous.
#KRYPTON MASS NUMBER FREE#
The periodic table includes 92 naturally occurring elements and 8 or more radioactive elements synthesized by nuclear reactions.įree element metals are found in free elemental form, e.g. Mass of 1 amu = 1.66 × 10 −27 kg.Įlements, chemical elements, are substances that cannot be separated into simpler substances.Īll matter consists of single elements or combinations of elements. The atomic mass unit is 1/12 of the mass of 12C. Ītomic mass units (u) are used to state the mass of an individual particle, e.g. See IUPAC Periodic Table of the Elements.

= 35.4527, so atomic weight is usually quoted as 35.5.įor atomic weight values listed for elements with no stable isotopes. See diagram 2.2.0: Carbon-12, Uranium-238, Oxygen-16Ītomic weight, relative atomic mass (r.a.m.), of an element, is the ratio of the average atomic mass of an atom of an element, including the common isotopes, to 1/12 the mass of an atom of carbon-12, the unified atomic mass unit.Īwt, Atomic weight - elements with no stable isotopesĬhlorine is 75% chlorine-35 and 25% chlorine-37, r.a.m. Mass number, A, atomic mass number, nucleon number, is the total number of protons and neutrons in the nucleus of an atom of an element.Ītomic number is shown in the left subscript position and mass number is shown in the left superscript position. They form part of the group of transition metals.Ītomic number, Z, is the number of protons in the nucleus of an atom of an element. Radiation, ionizing radiation, Geiger counter: 2.9.0Īctinoids, rare earth elements, from 89 Ac, to 103 Lr inclusive, are metals shown separately below the main table, in period P7a. Please send comments to: Terms applied to the Table of the Elements:


 0 kommentar(er)
0 kommentar(er)
